Anticorps > 20141 - Purified freeze-dried antibody to rat type I collagen
20141 - Purified freeze-dried antibody to rat type I collagen
Type I collagen is a fibrillar collagen composed of two identical chain α 1 (I) chains and one α 2 (I). Type I collagen is found in most connective tissues and is abundant in bone, cornea, dermis and tendon. Mutations in this gene are associated with osteogenesis imperfecta types I-IV, Ehlers-Danlos syndrome type VIIA, Ehlers-Danlos syndrome Classical type, Caffey Disease and idiopathic osteoporosis. [according to R. Dalgleish]
Immunogen : Type I collagen extracted from rat skin.
Host : Rabbit.
Polyclonal antibody purified by chromatography.
For research only.
Purified, freeze-dried antibody in 0.1 mL vials. Reconstitute with 0.1 mL distilled water and store aliquots at -20°C.
Liquid bulk of 5 mL. Aliquoted and stored at -20°C.
Unreconstituted
24 months at -20°C.
Reconstituted
Before use aliquot and store at -20°C (6 months). Avoid repeated freeze/thaw cycles.
Applications
IF, IHC, ELISA, SP(RIA).
Working dilutions
ELISA ≥ 1/2000 (OD = 0.5)
IF ≥ 1/40
IHC ≥ 1/500
Optimal dilutions should be determined by the end user.
- #404g
-
IgG
1.21
pH
7.31
ELISA
Optimal working dilution at 1/8000.
IF
Not tested.
IHC
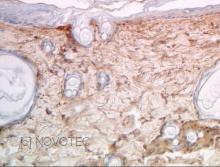
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on fixed paraffin-embedded rat skin.
-
- #480c
-
IgG
1.53
pH
7
ELISA
Optimal working dilution at 1/10000.
IF
Optimal working dilution not tested.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on fixed paraffin-embedded rat skin.
-
- #507b
-
IgG
1.31 mg/mL
pH
7.27
ELISA
Optimal working dilution at 1/6000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on fixed paraffin-embedded rat skin.
-
- #542f
-
IgG
3.42 mg/mL
pH
7.42
ELISA
Optimal working dilution at 1/3 500.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on fixed paraffin-embedded rat skin.
-
Presentation
Type I collagen is a fibrillar collagen composed of two identical chain α 1 (I) chains and one α 2 (I). Type I collagen is found in most connective tissues and is abundant in bone, cornea, dermis and tendon. Mutations in this gene are associated with osteogenesis imperfecta types I-IV, Ehlers-Danlos syndrome type VIIA, Ehlers-Danlos syndrome Classical type, Caffey Disease and idiopathic osteoporosis. [according to R. Dalgleish]
Description
Immunogen : Type I collagen extracted from rat skin.
Host : Rabbit.
Polyclonal antibody purified by chromatography.
For research only.
Format
Purified, freeze-dried antibody in 0.1 mL vials. Reconstitute with 0.1 mL distilled water and store aliquots at -20°C.
Liquid bulk of 5 mL. Aliquoted and stored at -20°C.
Stability
Unreconstituted
24 months at -20°C.
Reconstituted
Before use aliquot and store at -20°C (6 months). Avoid repeated freeze/thaw cycles.
Use
Applications
IF, IHC, ELISA, SP(RIA).
Working dilutions
ELISA ≥ 1/2000 (OD = 0.5)
IF ≥ 1/40
IHC ≥ 1/500
Optimal dilutions should be determined by the end user.
Lots
- #404g
-
IgG :
1.21
pH :
7.31
ELISA
Optimal working dilution at 1/8000.
IF
Not tested.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on fixed paraffin-embedded rat skin.
-
- #480c
-
IgG :
1.53
pH :
7
ELISA
Optimal working dilution at 1/10000.
IF
Optimal working dilution not tested.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on fixed paraffin-embedded rat skin.
-
- #507b
-
IgG :
1.31 mg/mL
pH :
7.27
ELISA
Optimal working dilution at 1/6000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on fixed paraffin-embedded rat skin.
-
- #542f
-
IgG :
3.42 mg/mL
pH :
7.42
ELISA
Optimal working dilution at 1/3 500.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on fixed paraffin-embedded rat skin.
-
References
- «"De novo generation in an in vivo rat model and biomechanical characterization of autologous transplants for ligament and tendon reconstruction. Clinical Biomechanics. 2017 Dec 14;52:33-40. "»
Soubeyranda M., Laemmel E., Maurel N., Diop A., Lazure T., Duranteau J., Vicaut E. - «Immunohistochemical study of collagens of the extracellular matrix in cartilage of Sepia officinalis. Eur. J. Histochem. 1999, 43, 211-225.»
Bairati A., Comazzi M., Gioria M., Hartmann D.J., Leone F., Rigo C. - «Extracellular Matrix Deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab. Invest. 1997, 76, 765-778.»
Desmoulière A., Darby I., Monte Alto Costa A., Raccurt M., Tuchweber B., Sommer P., Gabbiani G. - «Morphological and immunocytochemical characterization of cultured rat incisor cervical epithelial cells. Archs oral Biol. 1991, 36, 737-745.»
Farges J.C., Couble M.L., Joffre A., Hartmann D.J., Magloire H. - «Isolation and characterization of rat alveolar bone cells. Cell Mol. Biol. 1991, 37, 509-517.»
Bouvier M., Couble M.L., Hartmann D.J., Magloire H. - «Subpopulations of rat lung fibroblasts with different amounts of type I and type III collagen mRNAs. J. Biol. Chem. 1990, 265, 6286-6290.»
Breen E., Falco V.M., Absher M., Cutroneo K.R. - «Ultrastructural and immunocytochemical study of bone-derived cells cultured in three-dimensional matrices : influence of chondroitin-4 sulfate on mineralization. Differentiation 1990, 45, 128-137.»
Bouvier M., Couble M.L., Hartmann D.J., Gauthier J.P., Magloire H. - «Immunohistochemical study of the biological fate of a subcutaneous bovine collagen implant in rat. Histochemistry 1989, 91, 177-184.»
Vialle-Presles M.J., Hartmann D.J., Franc S., Herbage D. - «Differential immunohistochemical localization of cytokeratins and collagen types I and III in experimentally-induced cirrhosis. J. Pathol. 1989, 159, 151-158.»
Al Adnani M.S. - «Collagen immunotyping in human liver : Light and electron microscope study. J. Histochem. Cytochem. 1980, 28, 1145-1156.»
Grimaud J.A., Druguet M., Peyrol S., Chevalier O., Herbage D., El Badrawy N.