Anticorps > 20121 - Purified freeze-dried antibody to bovine type I collagen
20121 - Purified freeze-dried antibody to bovine type I collagen
Type I collagen is a fibrillar collagen composed of two identical chain α1(I) chains and one α2(I). Type I collagen is found in most connective tissues and is abundant in bone, cornea, dermis and tendon. Mutations in this gene are associated with osteogenesis imperfecta types I-IV, Ehlers-Danlos syndrome type VIIA, Ehlers-Danlos syndrome Classical type, Caffey Disease and idiopathic osteoporosis. [according to R. Dalgleish]
Immunogen : Type I collagen extracted from bovine skin.
Host : Rabbit.
Polyclonal antibody purified by chromatography.
For research only.
Purified, freeze-dried antibody in 0.1 mL vials. Reconstitute with 0.1 mL distilled water and store aliquots at -20°C.
Unreconstituted
24 months at -20°C.
Reconstituted
Before use aliquot and store at -20°C (6 months). Avoid repeated freeze/thaw cycles.
Applications
IF, IHC, ELISA, SP(RIA).
Working dilutions
ELISA ≥ 1/2000 (OD = 0.5)
IF ≥ 1/40
IHC ≥ 1/500
Optimal dilutions should be determined by the end user.
- #322m
-
IgG
2.95
pH
7.3
ELISA
Optimal working dilution at 1/30 000.
IF
Optimal working dilution not tested.
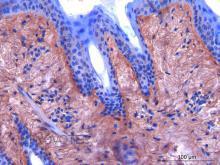
IHC
Immunoperoxidase technique with the Envision / Dako kit, hyaluronidase 0.5% pretreatment, optimal working dilution at 1/2000 on fixed paraffin-embedded bovine skin.
-
- #521a
-
IgG
2.91 mg/mL
pH
7.51
ELISA
Optimal working dilution at 1/10000.
IF
Optimal working dilution not tested.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5%hyaluronidase pretreatment, optimal working dilution at 1/1000 on fixed paraffin-embedded bovine skin.
-
- #533d
-
IgG
2.90 mg/mL
pH
7.23
ELISA
Optimal working dilution at 1/7000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/2000 on fixed paraffin-embedded bovine skin.
-
Presentation
Type I collagen is a fibrillar collagen composed of two identical chain α1(I) chains and one α2(I). Type I collagen is found in most connective tissues and is abundant in bone, cornea, dermis and tendon. Mutations in this gene are associated with osteogenesis imperfecta types I-IV, Ehlers-Danlos syndrome type VIIA, Ehlers-Danlos syndrome Classical type, Caffey Disease and idiopathic osteoporosis. [according to R. Dalgleish]
Description
Immunogen : Type I collagen extracted from bovine skin.
Host : Rabbit.
Polyclonal antibody purified by chromatography.
For research only.
Format
Purified, freeze-dried antibody in 0.1 mL vials. Reconstitute with 0.1 mL distilled water and store aliquots at -20°C.
Stability
Unreconstituted
24 months at -20°C.
Reconstituted
Before use aliquot and store at -20°C (6 months). Avoid repeated freeze/thaw cycles.
Use
Applications
IF, IHC, ELISA, SP(RIA).
Working dilutions
ELISA ≥ 1/2000 (OD = 0.5)
IF ≥ 1/40
IHC ≥ 1/500
Optimal dilutions should be determined by the end user.
Lots
- #322m
-
IgG :
2.95
pH :
7.3
ELISA
Optimal working dilution at 1/30 000.
IF
Optimal working dilution not tested.
IHC
Immunoperoxidase technique with the Envision / Dako kit, hyaluronidase 0.5% pretreatment, optimal working dilution at 1/2000 on fixed paraffin-embedded bovine skin.
-
- #521a
-
IgG :
2.91 mg/mL
pH :
7.51
ELISA
Optimal working dilution at 1/10000.
IF
Optimal working dilution not tested.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5%hyaluronidase pretreatment, optimal working dilution at 1/1000 on fixed paraffin-embedded bovine skin.
-
- #533d
-
IgG :
2.90 mg/mL
pH :
7.23
ELISA
Optimal working dilution at 1/7000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/2000 on fixed paraffin-embedded bovine skin.
-
References
- «Fresh and in vitro osteodifferentiated human amniotic membrane, alone or associated with an additional scaffold, does not induce ectopic bone formation in Balb/c mice. Cell and Tissue Banking, 1-9»
Laurent R., Nallet A., De Billy B., Obert L., Nicod L., Meyer C., Layrolle P., Zwetyenga N., Gindraux F. - «Storage and qualification of viable intact human amniotic graft and technology transfer to a tissue bank. Cell Tissue Bank. 2014 Jun;15(2):267-75.»
Laurent R., Nallet A., Obert L., Nicod L., Gindraux F. - «Influence of medium composition, static and stirred conditions on the proliferation of and matrix protein expression of bovine articular chondrocytes cultures in 3-D collagen scaffold. Biomaterial 2004, 25, 687-697.»
Freyria A.M., Cortial D., Ronzière M.C., Guerret S., Herbage D. - «Type V regulates the assembly of collagen fibrils in cultures of bovine vascular smooth muscle cells. J. Cell. Biochem. 2000, 80, 146-155.»
Kypreos K.E., Birk D., Trinkaus-Randall V., Hartmann D.J., Sonenshein G.E. - «Expansion of cortical interstitium is limited by converting enzyme inhibition in type 2 diabetic patients with glomerulosclerosis. J. Am. Soc. Nephrol. 1999, 10, 1253-1263.»
Cordonnier D.J., Pinel N., Barro C., Maynard C., Zaoui P., Halimi S., Hurault de Ligny B., Reznic Y., Simon D, Bilous R.W. - «Analysis of types I, II, III, IX and XI collagens synthesized by fetal bovine chondrocytes in high-density culture. Osteoarthritis Cart. 1997, 5, 205-214.»
Ronziere M.C., Farjanel J., Freyria A.M., Hartmann D.J., Herbage D. - «Lysyl oxidase gene expression in the stromal reaction to in situ and invasive ductal breast carcinoma. Am. J. Pathol. 1997, 150, 497-507.»
Peyrol S., Raccurt M., Gerard F., Gleyzal C., Grimaud J.A., Sommer P. - «Analysis of collagens solubilized from cartilage of normal and spontaneously osteoarthritic rhesus monkeys. Osteoarthritis and Cart. 1994, 2, 227-234.»
Grympas M.D., Gahunia H.K., Yuan J., Pritzker K.P.H., Hartmann D.J., Tupy J.H. - «Composition and organization of the collagen network produced by fetal bovine chondrocytes cultured at high density. J. Histochem. Cytochem. 1993, 41, 867-875.»
Ruggiero F., Petit B., Ronzière M.C., Farjanel J., Hartmann D.J., Herbage D. - «Structural and compositional divergencies in the extracellular matrix encountered by neural crest cells in the white mutant axolotl embryo. Development 1990, 109, 533-551.»
Perris R., Löfberg J., Fällström C., Von Boxberg Y., Olsson L., Newgreen D.F. - «Physiological behaviour of rabbit articular chondrocytes immobilized in a calcium alginate gel. In : Physiology of immobilized cells, J.A.M.de Bont, Tramper J., Hamer G., Harder W., Mattiasson B. Eds, Elsevier Science PublImhers B.V., Amsterdam, 1990.»
Guyot J.B., Tamponnet C., Lièvremont M. - «Immunohistochemical study of the biological fate of a subcutaneous bovine collagen implant in rat. Histochemistry 1989, 91, 177-184.»
Vialle-Presles M.J., Hartmann D.J., Franc S., Herbage D. - «Rabbit articular chondrocytes : an in vitro model for studying the effect of sodium auro-thiopropanol sulfonate on proliferation kinectics, type II collagen phenotype and mitochondrial activity. Fundam. Clin.Pharmacol. 1988, 2,57-67.»
Ronot X., Hainque B., ChrImten M.O., Froger B., Hartmann D.J., Adolphe M., Lechat P. - «Collagen heterogeneity in bovine and human cartilage. In : Osteoarthritis-Current clinical and fundamental problems, Peyron J.G. Ed. Primard, Paris, 1985, 123-133.»
Ricard-Blum S., Tiollier J., Dodille M.P., Hartmann D.J., Magloire H., Garrone R., Ville G., Herbage D. - «Biochemical properties and immunolocalization of minor collagens in foetal calf cartilage. FEBS Letters 1982, 146, 343-347.»
Ricard-Blum S., Hartmann D.J., Herbage D., Payen-Meyran C., Ville G.