Antibody > 20451 - Purified freeze-dried antibody to mouse type IV collagen
20451 - Purified freeze-dried antibody to mouse type IV collagen
Type IV collagen is a sheet-forming type of collagen found in basement membranes where it serves a critical scaffolding function. In mammals, six proteins make up the type IV collagen family with the distribution of family members being tissue-specific. Structurally, three collagen IV alpha chains assemble to form a flexible heterotrimeric protomer that can self-assemble into network polymers. Tissue injury in the autoimmune disease Goodpasture syndrome is due to pathogenic autoantibodies targeting the Collagen IV α3 chain. Mutations in COL4A5 are associated with Alport syndrome. [according to RefSeq]
Immunogen : Type IV collagen extracted from mouse tumor tissues.
Host : Rabbit.
Polyclonal antibodies purified by chromatography.
For research only.
Purified, freeze-dried antibody in 0.1 mL vials. Reconstitute with 0.1 mL distilled water and store aliquots at -20°C.
Liquid bulk of 5 mL. Aliquoted and stored at -20°C.
Unreconstituted
24 months at -20°C.
Reconstituted
Before use aliquot and store at -20°C (6 months). Avoid repeated freeze/thaw cycles.
Applications
IF, IHC, ELISA, SP(RIA).
Working dilutions
ELISA ≥ 1/2000 (OD = 0.5)
IF ≥ 1/40
IHC ≥ 1/500
Optimal dilutions should be determined by the end user.
- #544b
-
IgG
1.34mg/mL
pH
7.44
ELISA
Optimal working dilution at 1/20 000.
IF
Optimal working dilution not tested with our current protocol.
IHC
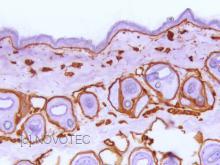
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on frozen mouse skin.
-
- #409g
-
IgG
1.62 mg/mL
pH
7.17
ELISA
Optimal working dilution at 1/60 000 (DO:0.5).
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/4000 on frozen mouse skin.
-
- #435h
-
IgG
0.885 mg/mL
pH
7.33
ELISA
Optimal working dilution at 1/40000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, optimal working dilution at 1/4000 on frozen mouse skin.
-
- #461g
-
IgG
1.11 mg/mL
pH
7.47
ELISA
Optimal working dilution at 1/40000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, optimal working dilution at 1/2000 on frozen mouse skin.
-
- #484d
-
IgG
1.21 mg/mL
pH
7.34
ELISA
Optimal working dilution at 1/40 000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5 hyaluronidase pretreatment, optimal working dilution at 1/2000 on paraffin-embedded mouse skin.
-
- #501d
-
IgG
1.44 mg/mL
pH
7.34
ELISA
Optimal working dilution at 1/40000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/2000 on fixed paraffin-embedded mouse skin.
-
- #518d
-
IgG
1.40 mg/mL
pH
7.39
ELISA
Optimal working dilution at 1/30000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on frozen paraffin-embedded mouse skin.
-
- #534c
-
IgG
1.43 mg/mL
pH
7.49
ELISA
Optimal working dilution at 1/10000.
IF
Optimal working dilution not tested.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on frozen mouse skin.
-
- #536b
-
IgG
3.75 mg/mL
pH
7.7
ELISA
Optimal working dilution at 1/40 000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on unfixed frozen mouse skin.
-
- #536c
-
IgG
3.75 mg/mL
pH
7.7
ELISA
Optimal working dilution at 1/40000.
IF
Optimal dilution not tested.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on frozen mouse skin.
-
- #552j
-
IgG
1.12 mg/mL
pH
7.33
ELISA
Optimal working dilution at 1/25 000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on frozen mouse skin.
-
- #555d
-
IgG
1.25 mg/mL
pH
7.42
ELISA
Optimal working dilution at 1/20000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/2000 on frozen mouse skin.
-
- #556c
-
IgG
1.21 mg/mL
pH
7.45
ELISA
Optimal working dilution at 1/25000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/2000 on frozen mouse skin.
-
- #560j
-
IgG
1.55mg/mL
pH
7.22
ELISA
Optimal working dilution at 1/20000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/2000 on frozen mouse skin.
-
Presentation
Type IV collagen is a sheet-forming type of collagen found in basement membranes where it serves a critical scaffolding function. In mammals, six proteins make up the type IV collagen family with the distribution of family members being tissue-specific. Structurally, three collagen IV alpha chains assemble to form a flexible heterotrimeric protomer that can self-assemble into network polymers. Tissue injury in the autoimmune disease Goodpasture syndrome is due to pathogenic autoantibodies targeting the Collagen IV α3 chain. Mutations in COL4A5 are associated with Alport syndrome. [according to RefSeq]
Description
Immunogen : Type IV collagen extracted from mouse tumor tissues.
Host : Rabbit.
Polyclonal antibodies purified by chromatography.
For research only.
Format
Purified, freeze-dried antibody in 0.1 mL vials. Reconstitute with 0.1 mL distilled water and store aliquots at -20°C.
Liquid bulk of 5 mL. Aliquoted and stored at -20°C.
Stability
Unreconstituted
24 months at -20°C.
Reconstituted
Before use aliquot and store at -20°C (6 months). Avoid repeated freeze/thaw cycles.
Use
Applications
IF, IHC, ELISA, SP(RIA).
Working dilutions
ELISA ≥ 1/2000 (OD = 0.5)
IF ≥ 1/40
IHC ≥ 1/500
Optimal dilutions should be determined by the end user.
Lots
- #544b
-
IgG :
1.34mg/mL
pH :
7.44
ELISA
Optimal working dilution at 1/20 000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on frozen mouse skin.
-
- #409g
-
IgG :
1.62 mg/mL
pH :
7.17
ELISA
Optimal working dilution at 1/60 000 (DO:0.5).
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/4000 on frozen mouse skin.
-
- #435h
-
IgG :
0.885 mg/mL
pH :
7.33
ELISA
Optimal working dilution at 1/40000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, optimal working dilution at 1/4000 on frozen mouse skin.
-
- #461g
-
IgG :
1.11 mg/mL
pH :
7.47
ELISA
Optimal working dilution at 1/40000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, optimal working dilution at 1/2000 on frozen mouse skin.
-
- #484d
-
IgG :
1.21 mg/mL
pH :
7.34
ELISA
Optimal working dilution at 1/40 000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5 hyaluronidase pretreatment, optimal working dilution at 1/2000 on paraffin-embedded mouse skin.
-
- #501d
-
IgG :
1.44 mg/mL
pH :
7.34
ELISA
Optimal working dilution at 1/40000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/2000 on fixed paraffin-embedded mouse skin.
-
- #518d
-
IgG :
1.40 mg/mL
pH :
7.39
ELISA
Optimal working dilution at 1/30000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on frozen paraffin-embedded mouse skin.
-
- #534c
-
IgG :
1.43 mg/mL
pH :
7.49
ELISA
Optimal working dilution at 1/10000.
IF
Optimal working dilution not tested.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on frozen mouse skin.
-
- #536b
-
IgG :
3.75 mg/mL
pH :
7.7
ELISA
Optimal working dilution at 1/40 000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on unfixed frozen mouse skin.
-
- #536c
-
IgG :
3.75 mg/mL
pH :
7.7
ELISA
Optimal working dilution at 1/40000.
IF
Optimal dilution not tested.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on frozen mouse skin.
-
- #552j
-
IgG :
1.12 mg/mL
pH :
7.33
ELISA
Optimal working dilution at 1/25 000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/1000 on frozen mouse skin.
-
- #555d
-
IgG :
1.25 mg/mL
pH :
7.42
ELISA
Optimal working dilution at 1/20000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/2000 on frozen mouse skin.
-
- #556c
-
IgG :
1.21 mg/mL
pH :
7.45
ELISA
Optimal working dilution at 1/25000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/2000 on frozen mouse skin.
-
- #560j
-
IgG :
1.55mg/mL
pH :
7.22
ELISA
Optimal working dilution at 1/20000.
IF
Optimal working dilution not tested with our current protocol.
IHC
Immunoperoxidase technique with the Envision / Dako kit, 0.5% hyaluronidase pretreatment, optimal working dilution at 1/2000 on frozen mouse skin.
-
References
- «HANAC Col4a1 Mutation in Mice Leads to Skeletal Muscle Alterations due to a Primary Vascular Defect.»
Guiraud S., Migeon T., Ferry A., Chen Z., Ouchelouche S., Verpont M-C, Sado Y, Allamand V, Ronco P., Plaisie E.- Official Journal of the American Society for investigative pathology - article in press - «A human-mouse chimera of the alpha3 alpha4 alpha 5 (IV) collagen promoter rescues the renal phenotype in Col4a3-/-Alport mice. Am J Pathol 2003, 163, 1633-1644.»
Heidet L., Borza D.B., Jouin M., Sich M., Mattei M.G., Sado Y., Hudson B.G., Hastie N., Antignac C., Gubler M.C. - «Long term remodeling of a bilayered living human skin equivalent (Apligraf®) grafted onto nude mice: immunolocalization of human cells and characterization of extracellular matrix. Wound Rep. Reg. 2003, 11, 35-45.»
Guerret S., Govignon E., Hartmann D.J., Ronfard V. - «Expansion of cortical interstitium is limited by converting enzyme inhibition in type 2 diabetic patients with glomerulosclerosis. J. Am. Soc. Nephrol. 1999, 10, 1253-1263.»
Cordonnier D.J., Pinel N., Barro C., Maynard C., Zaoui P., Halimi S., Hurault de Ligny B., Reznic Y., Simon D, Bilous R.W. - «Pituitary hormones modulate cell-cell interactions between thymocytes and thymic epithelial cells. J. Neuroimmunol. 1997, 76, 39-49.»
De Mello-Coelho V., Villa-Verde D.M.S., Dardenne M., Savino W. - «In vivo and in vitro expression of tenascin by human thymic microenvironmental cells. Dev. Immunol. 1995, 4, 139-147.»
Sondermann-Freitas C., O'Campo-Lyra S.P., Dalmau S.R., Savino W. - «Increased deposition of extracellular matrix components in the thymus gland of MDX mouse: correlation with the muscular lesion. J. Neuroimmunology 1995, 59, 9-18.»
Quirico-Santos T., Ribeiro M.M., Savino W. - «Extracellular matrix components of the mouse thymus microenvironment. IV . Modulation of thymic nurse cells by extracellular matrix ligands and receptors. Eur. J. Immunol. 1994, 24, 659-664.»
Villa-Verde D.M.S., Machado J., Candido L., Vannier-Santos M.A., Chammas R., Brentani R.R., Savino W. - «Altered deposition of extracellular matrix components in the skeletal muscle and lymph node of the MDX dystrophic mouse. Brazilian J. Med. Biol. Res. 1994, 27, 2229-2240.»
Seixas S.I.L., Wajsenzon I.J., Savino W., Quirico-Santos T. - «Pleiotropic influence of triiodothyronine on thymus physiology. Endocrinology 1993, 133, 867-875.»
Villa-Verde D.M.S., De Mello-Coelho V., Farias De Oliveira D.A., Dardenne M., Savino W. - «The role of fibroblasts in dermal vascularization and remodeling of reconstructed human skin after transplantation onto the nude mouse. Transplantation 1992, 54, 317-326.»
Demarchez M., Hartmann D.J., Regnier M., Asselineau D. - «Origin of basement membrane type IV collagen in xenografted human epithelial tumor cells lines. Am. J. Pathol. 1990, 136, 1165-1172.»
Cleutjens J.P.M., Havenith M.G., Beek C., Vallinga M., Ten Kate J., Bosman F.T. - «Interferon-induced glomerular basement membrane and endothelial cell lesions in mice. An immunogold ultrastructural study of basement membrane components. Am. J. Pathol. 1988, 133, 557-563.»
Moss J., Shore I., Woodrow D., Gresser I. - «The revascularization process of human skin transplanted onto the nude mouse. In : Progress in Basement Membrane Research. Renal and Related Aspects in Health and Disease. Gubler M.C., Sternberg M. Eds. John Libbey Eurotext, London 1988, 293-294.»
Démarchez M., Hartmann D.J., Pruniéras M. - «Immunofluorescent localization of collagen types I, III and IV, fibronectin, laminin, and basement membrane proteoglycan in developing mouse skin. Roux's Arch. Dev. Biol. 1987, 196, 295-302.»
Mauger A., Emonard H., Hartmann D.J., Foidart J.M., Sengel P. - «An immunohistological study of the revascularization process in human skin transplanted onto the nude mouse. Transplantation 1987, 43, 896-903.»
Demarchez M., Hartmann D.J., Prunieras M. - «Wound-healing of human-skin transplanted onto the nude-mouse. 2. an immunohistological and ultrastructural study of the epidermal basement-membrane zone reconstruction and connective-tissue reorganization. Dev. Biol. 1987, 121, 119-129.»
Demarchez M., Hartmann D.J., Herbage D., Ville G., Prunieras M. - «Immunofluorescent localization of collagen types I, III, IV, fibronectin and laminin during morphogenesis of scales and scaleless skin in the chick embryo. Roux's Arch. Dev. Biol. 1983, 192, 205-215.»
Mauger A., Demarchez M., Herbage D., Grimaud J.A., Druguet M., Hartmann D.J. Foidart J.M., Sengel P. - «Répartition du collagène, de la fibronectine et de la laminine au cours de la morphogénèse de la peau et des phanères chez l'embryon de poulet. C. R. Acad. Sc. Paris 1982, 294, 475-480.»
Mauger A., Demarchez M., Georges D., Herbage D., Grimaud J.A., Druguet M., Hartmann D.J., Sengel P.